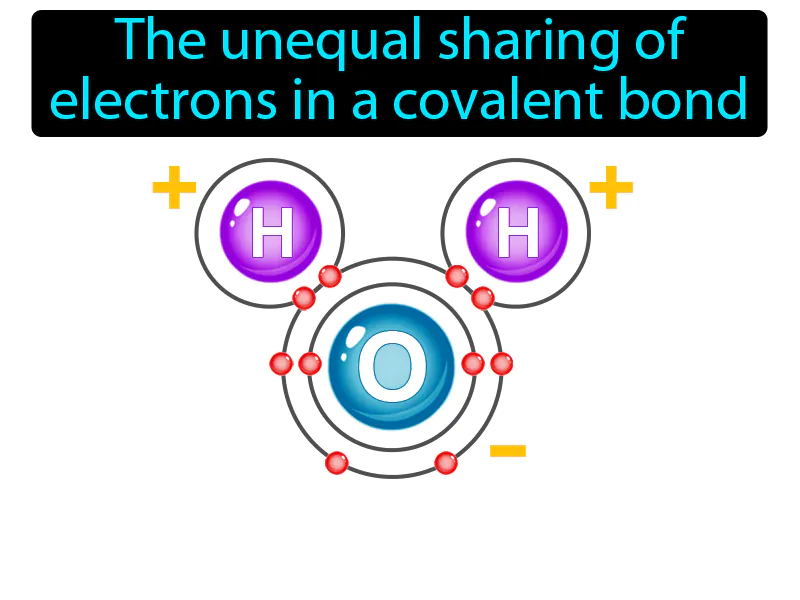
Polar Bond
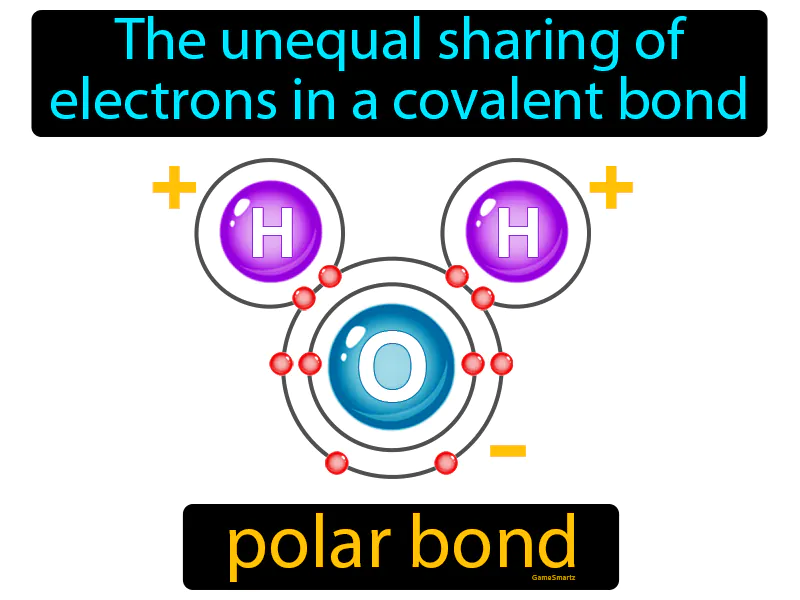
The unequal sharing of electrons in a covalent bond
Real World Example
Imagine you're sharing a pizza with a friend, but your friend loves pizza so much that they end up taking more slices than you. This is similar to a polar covalent bond, where two atoms share electrons, but one atom pulls the electrons closer to itself, much like your friend takes more pizza. In this analogy, the pizza represents the shared electrons, and your friend's stronger desire for pizza is like the stronger electronegativity of the atom that draws the electrons nearer, resulting in the unequal sharing characteristic of a polar bond.
Practice Version