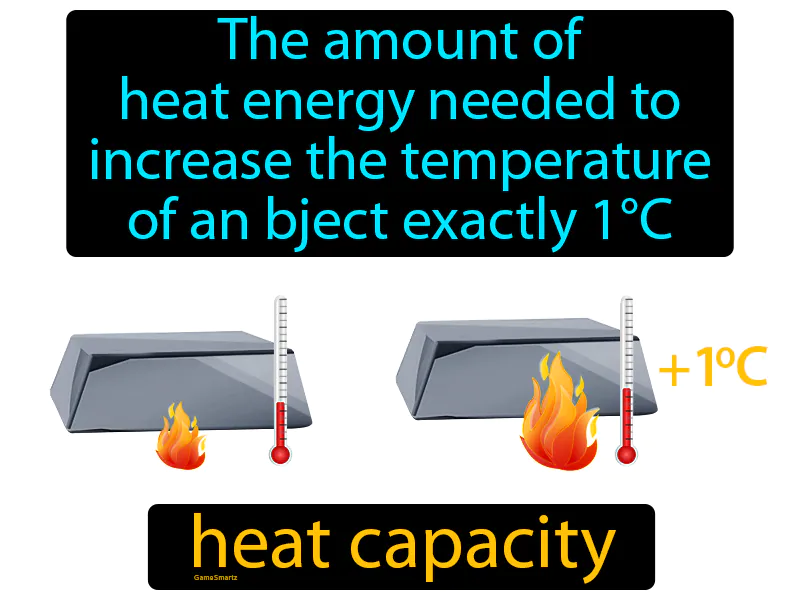
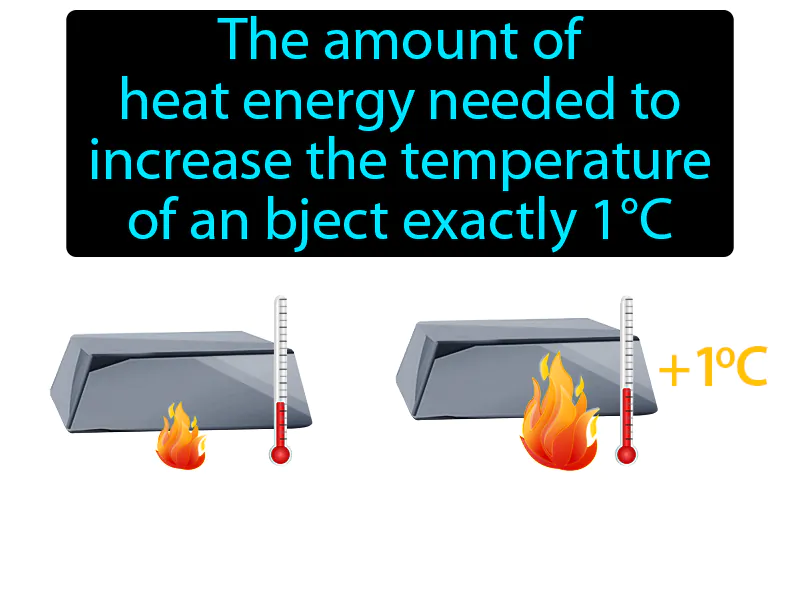
Heat Capacity
Loading image...
The amount of heat energy needed to increase the temperature of an object exactly 1°C
Real World Example
Imagine trying to fill a backpack with books for a trip. Just like each book adds weight to your backpack, each unit of heat energy increases the temperature of an object. The heat capacity is like the strength of the backpack straps; the stronger they are, the more weight (or heat energy) you can add before it feels too heavy (or increases the temperature by 1°C).
Practice Version
Loading image...